 Pseudomonas aeruginosa is an opportunistic pathogen that is the predominant cause of cystic fibrosis mortality. It is particularly hard to treat with antibiotics, due to forming thick layers of matrices within the sticky mucous of patient lungs, also known as biofilms. Formation of these biofilms is vastly advantageous for the survival and persistence of the bacteria but greatly reduces patient recovery from infection. Attempting to inhibit this layer formation would disarm the bacteria and make them increasingly susceptible to antibiotics that are readily available.
Pseudomonas aeruginosa is an opportunistic pathogen that is the predominant cause of cystic fibrosis mortality. It is particularly hard to treat with antibiotics, due to forming thick layers of matrices within the sticky mucous of patient lungs, also known as biofilms. Formation of these biofilms is vastly advantageous for the survival and persistence of the bacteria but greatly reduces patient recovery from infection. Attempting to inhibit this layer formation would disarm the bacteria and make them increasingly susceptible to antibiotics that are readily available.
This article investigates whether addition of a peptide could prevent the pathway that upregulates the biofilm formation, particularly the amidase operon. The amidase protein is only produced when the response regulator (AmiR) is free to bind to the operon. However, in increased biofilm formation stages the AmiR protein is bound to AmiC, preventing this association. Therefore, we hypothesised that by binding alternative peptides to AmiC we could increase production of the amidase and therefore decrease biofilm formation.
By combining Structural Bioinformatics with Microbiology, we showed that the peptide Osteocrin can bind to AmiC and decrease the biofilm thickness. The structural biology indicates that Osteocrin might bind to an alternative pocket of the protein, compared to our previously studied peptides, explaining the variation in activity. Therefore, future work will focus on obtaining structures of the complexes and using our knowledge to inform future therapeutic design.
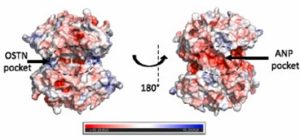
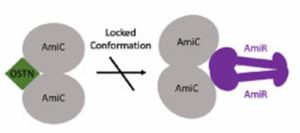
Osteocrin binds to the opposite side of the protein, compared to the previously studied Atrial Natriuretic Peptide (ANP). This prevents movement of the AmiC protein and therefore it cannot interact effectively with AmiR.
Courtney Lendon, SWBio DTP student
